-
Porous and Dense Magnesium Borohydride Frameworks: Synthesis, Stability, and Reversible Absorption of Guest Species

Y. Filinchuk, B. Richter, T.R. Jensen, V. Dmitriev, D. Chernyshov and H. Hagemann
Angewandte Chemie International Edition, 50 (47) (2011), p11162-11166


DOI:10.1002/anie.201100675 | unige:17480 | Abstract | Article PDF

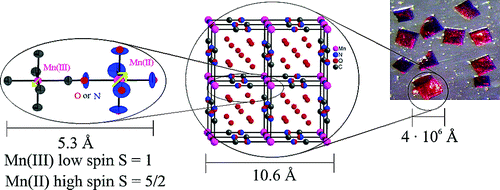
Highly occupied: A highly porous form of Mg(BH4)2 (see picture; Mg green, BH4 blue, unit cells shown in red) reversibly absorbs H2, N2, and CH2Cl2. At high pressures, this material transforms into an interpenetrated framework that has 79 % higher density than the other polymorphs. Mg(BH4)2 can act as a coordination polymer that has many similarities to metal–organic frameworks.